The Erwin-Schrodinger electron cloud model, also known as the Schrödinger wave equation, was developed in 1926 to describe the behavior of electrons in atoms. Schrödinger proposed to describe the electron as a wavefunction existing in his three-dimensional space surrounding the nucleus.
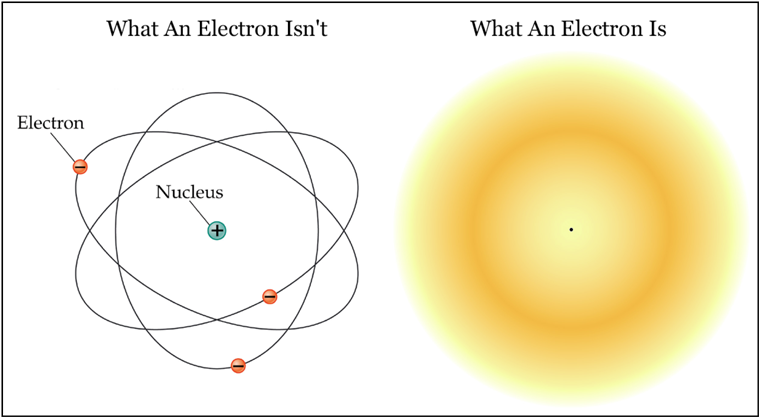
This model assumes that electrons move around the nucleus in ways best described by probability distributions or electron clouds, rather than specific paths.
Limitations of Electron Cloud Model:
The Schrödinger electron cloud model has revolutionized our understanding of atomic structure by allowing scientists to more accurately predict the behavior and properties of electrons within atoms.
This model was based on mathematical formulas that help explain how electrons are distributed in energy levels or orbitals around the nucleus. Schrödinger’s work paved the way for the development of modern quantum mechanics.
Quantum mechanics is now used to describe the behavior of matter at the atomic and subatomic levels.
Electron cloud models have come a long way, but have some limitations. One of the main drawbacks of this model is that it assumes that the electrons are in steady state. However, electrons in atoms are in constant motion, and it is difficult to predict this movement accurately.
Moreover, this model does not consider the effects of external forces on electron behavior, such as the effects of applied electric or magnetic fields. Another limitation of electron cloud models is their inability to accurately describe electron behavior in more complex atoms or molecules.
In such cases, the model becomes increasingly difficult to apply and more sophisticated techniques are required to predict electron behavior.
Electron cloud model vs. quantum mechanics model:
Both the electron cloud model and the quantum mechanics model are used to describe the behavior of electrons in atoms, but there are some important differences between them. The quantum mechanical model is a more sophisticated version of the electron cloud model that considers wave-particle duality of electrons.
It describes the behavior of electrons in a mathematical formula known as the wavefunction, which predicts the probability of finding an electron at a particular location. Unlike electron cloud models, quantum mechanical models can be applied to more complex systems, including molecules and solids.
It also more accurately describes the behavior of electrons in atoms with multiple electrons. Quantum mechanical models are essential to the development of modern technologies, including the development of transistors and lasers.
How does the electron cloud model describe electrons?
The electron cloud model describes electrons as existing in energy levels or orbitals around the nucleus. These orbitals are defined by a set of quantum numbers that describe the size, shape, and orientation of the electron cloud.
This model assumes that electrons exist in a stochastic cloud that reflects the probability of finding an electron at a particular location. Electron clouds are created by the electromagnetic force between positively charged nuclei and negatively charged electrons. Electrons can exist in any of four main types of orbitals.
s, p, d, or f. The shape of these orbitals determines the energy of the electron, which is related to its behavior in chemical reactions. In summary, the electron cloud model provides a stochastic view of the behavior of electrons in atoms, describing electrons as wave-like entities that exist in three-dimensional space around the nucleus.
Despite some limitations, the electron cloud model is of great importance in the development of modern atomic theory and in understanding how matter behaves at the atomic and subatomic level.
